Helping new nasal swab tests to become available to the public for quick and easy diagnosis of viruses such as COVID, Flu, and RSV.
Ensure Timely Detection
Early detection is the key to managing symptoms and preventing the spread of severe respiratory illnesses like COVID-19, RSV, and the Flu in Central Illinois. These viruses can cause severe illness, especially in vulnerable populations. They often share similar symptoms, making precise diagnostics crucial.
Koch Research, a leader in medical research, conducts diagnostic studies to develop and refine nasal swab tests for COVID-19, RSV, and the Flu. Our team of experts designed these tests to quickly and accurately identify each virus, ensuring timely treatment and reducing the risk of transmission. Your participation in these studies will not only improve public health but also contribute to the safety of our communities.
Risk Factors for Severe Respiratory Illness
- Age (young children and older adults)
- Chronic respiratory conditions
- Weakened immune system
- Cardiovascular diseases
- Smoking and lifestyle factors
Disclaimer
**Participation in the study, including all procedures and treatment, including the investigational drug, is provided at no cost, and having insurance is not required**
Enroll Now!
Interested in participating in a clinical trial?
COVID-19, Flu, and RSV Diagnostic Clinical Trials
At Clinical Research, our diagnostic trials for COVID-19, flu, and RSV are designed to be efficient, straightforward, and comfortable. Each trial involves two simple nasal swabs, with the entire process typically taking no more than 30 minutes.
Eligibility Criteria
- Age: 6 months or older
- Gender: Male & Female
- Condition: Cold/flu symptoms
- Status : Not Recruiting
Nasal Swab: Your Key to Accurate, Early Diagnosis
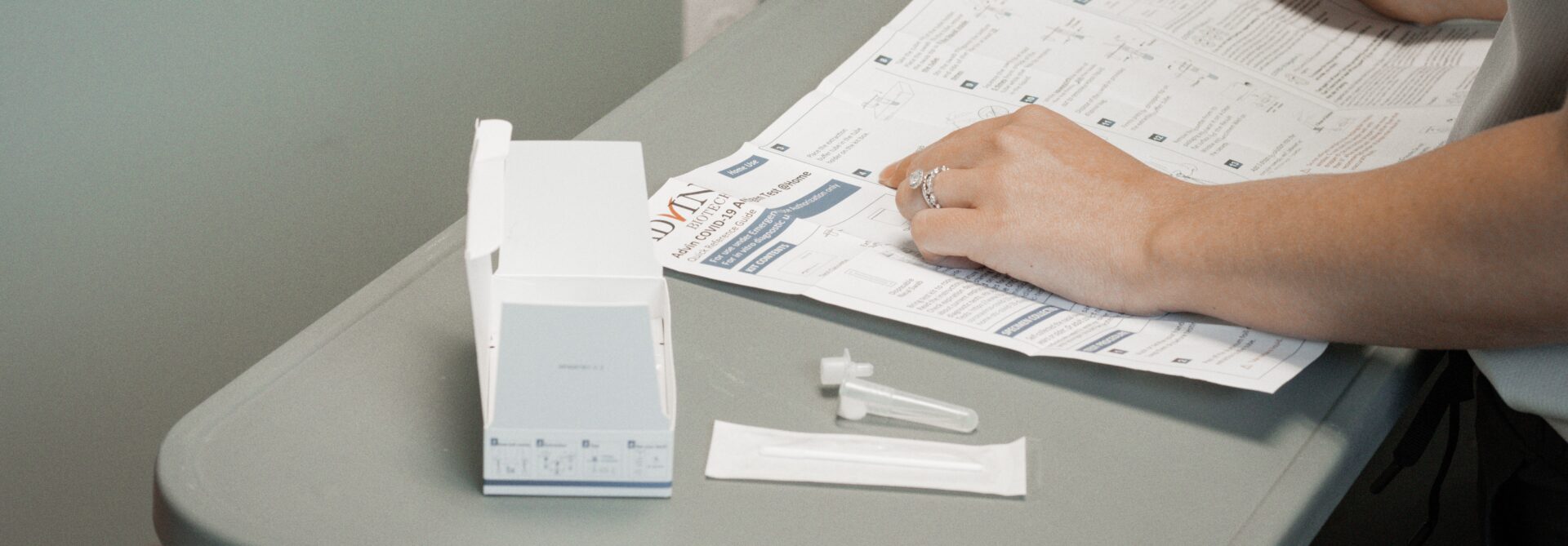
The nasal swab test is a quick and simple diagnostic tool for respiratory infections like COVID-19, RSV, and the flu. This noninvasive procedure involves gently swabbing the inside of your nose to collect a sample. It’s a straightforward process that can be done quickly and comfortably, helping healthcare providers identify the exact cause of your symptoms.
Nasal swabs are highly effective for early detection, allowing doctors to diagnose infections before they worsen or spread to others. With swift results, treatment can begin sooner, potentially reducing the severity of illness and protecting those around you.
Whether you’re feeling unwell or need routine testing, nasal swabs offer a reliable, fast, and easy way to get the answers you need for your health. They are a safe and convenient tool in healthcare diagnostics, helping you stay informed and protected.
What is the process for participating in nasal swab study at Koch Clinical Research?
- Let us know you are interested by calling or texting us at 309-291-0585 or use the form above to sign up online.
- Someone from the Koch Clinical Research Staff will contact you to review the criteria to join the study and to go over the study procedures.
- If you qualify for the study, we will schedule an appointment with you to participate in the study at our office.
- The first thing that will happen during your appointment will be to go over an “Informed Consent” document which will outline the study procedures and will require your signature before we can continue.
- Our staff will review the criteria to join the study (again) to make sure you qualify before collecting any other study-related information or samples.
- We will collect any required information and nasal swab samples. For some studies, you will collect the nasal swab sample and perform the test yourself so that you can provide feedback on its ease-of-use.
- Nasal swab studies only require a single visit, taking 30-60 minutes, and will not require any follow-up visits. At the conclusion of your visit, you will be compensated for your time and travel.
Frequently Asked Questions
How does a new nasal swab testing device get FDA approval to be sold?
In order to be approved for use, a new nasal swab test must prove that it is safe and accurate. The FDA (Food and Drug Administration) requires the new nasal swab testing device to be tested at multiple clinical research offices, which is why we need volunteers like you. We usually look for people with current cold or flu symptoms to collect data that will determine if the device can accurately detect the viruses being tested.
How long does it take to get results from a nasal swab test?
The rapid nasal swab tests that are being studied for efficiency and accuracy in our office usually give results in 15-30 minutes. Other nasal swab tests may take 24-72 hours.
Are nasal swabs painful?
Nasal swabs can cause mild discomfort or a tickling sensation, but are generally not painful. The swabbing process is quick and usually lasts 5-10 seconds.
Can nasal swabs detect multiple viruses at once?
Yes, some nasal swab tests are designed to detect multiple viruses, such as COVID-19, RSV, and the flu, in a single sample, making it more efficient for diagnosing respiratory illnesses.
How accurate are nasal swab tests?
Nasal swab tests are not perfectly accurate, and some tests can change in accuracy as viruses mutate, however the FDA reviews accuracy data for any new test before it can be sold. This is why volunteers are needed to test these devices before they are approved to be sold to the public.