Conducting COVID-19 and Flu Clinical Trials to discover potential vaccines that protects against both viruses.
Build a Stronger Defense
Influenza affects millions globally each year, while COVID-19 has led to a pandemic and still impacts public health. Influenza and COVID-19 are highly contagious diseases with symptoms like body aches, fever, and cough. Combining vaccines for both viruses could simplify care and benefit both patients and caregivers
Understanding the need for immunity, Koch Research flu clinical trials aim to assess the safety and efficacy of a dual influenza and COVID-19 vaccine, comparing it to separate vaccines for protection against influenza and SARS-CoV-2.
Risk Factors
- Age
- Chronic health conditions
- Pregnancy
- Weakened immune system
- Obesity
Disclaimer
**Participation in the study, including all procedures and treatment including the investigational drug, is provided at no cost, and having insurance is not required**
Enroll Now!
Interested in participating in a clinical trial?
Covid-19 and Flu Clinical Trials in Central Illinois
This clinical study evaluates the safety and efficacy of influenza and COVID-19 vaccine. By comprehensively examining these aspects, we aim to gain valuable insights into this condition and enhance protection against influenza and SARS-CoV-2. Ultimately, this research will contribute to boosting immunity against ongoing COVID-19 and flu pandemics.
Eligibility Criteria
- Age: 18 years or older
- Gender: Male & Female
- Condition: Healthy Person
- Status: Not Recruiting
Understanding Two Respiratory Threats
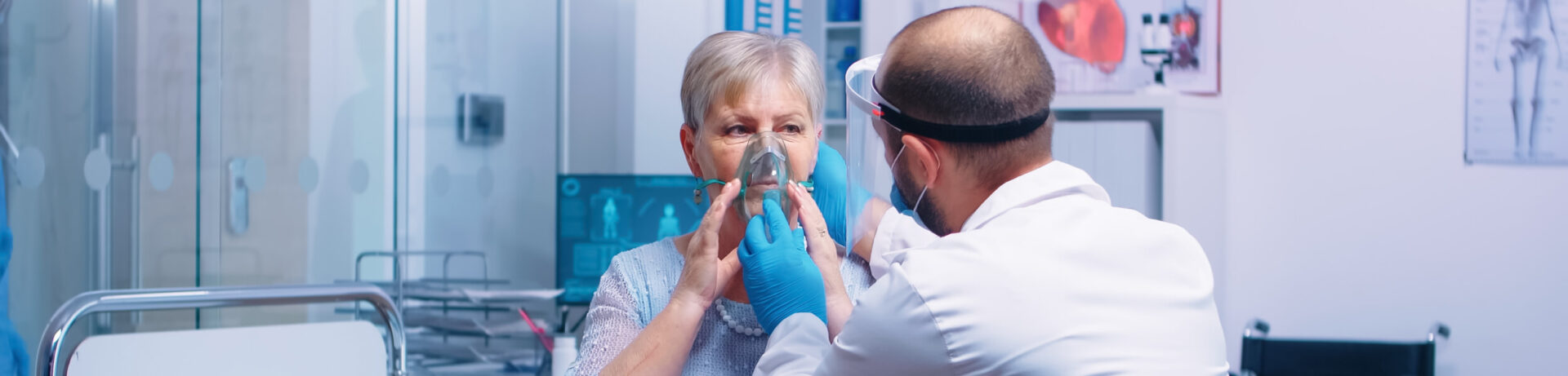
COVID-19 and influenza are commonly known as the flu. They are both respiratory illnesses caused by viruses. While COVID-19 is caused by the novel coronavirus SARS-CoV-2, the flu is caused by influenza viruses.
Both illnesses share similarities in symptoms such as fever, cough, and fatigue, although COVID-19 can cause more severe respiratory complications in some cases. The global COVID-19 pandemic has resulted in millions of deaths worldwide and continues to impact communities globally.
In contrast, the flu infects millions annually but typically has lower mortality rates. Vaccination, hygiene practices, and public health measures are crucial in preventing the spread of both viruses and reducing their impact on public health.
Koch Research’s COVID-19 vaccine clinical trials aim to manage both viruses with the vaccine.
Frequently Asked Questions
How long will my participation take?
It depends on the trial, but most of our vaccine trials have 2-3 follow up visits up to several months after you receive your vaccination to discuss any symptoms since receiving the vaccine.
Why participate in a combined vaccine trial?
At Koch Clinical Research, there may be an opportunity to participate in a vaccine trial which tests a two in one combination vaccine. You could help develop a vaccine that protects against two diseases at once, simplifying prevention strategies.
What if I've already gotten a COVID or flu shot?
Eligibility varies per trial, but most vaccine studies will allow previous vaccinations depending on how recently they were received. Contact us for more information.