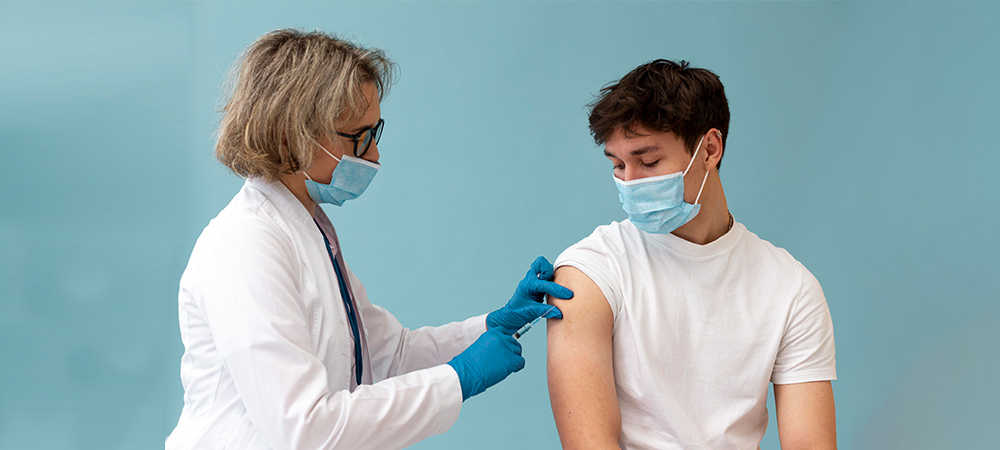
Healthy volunteers play a crucial role in vaccine trials by helping researchers assess the safety and effectiveness of new vaccines before approval. Their participation allows scientists to gather essential data on how the vaccine works in a diverse population. This helps ensure that vaccines are safe and effective for widespread use.
Vaccine development is a complex and methodical process. With each phase designed to gather crucial data on a vaccine’s safety, effectiveness, and potential side effects. Among these stages, the phase 2 vaccine trial is particularly significant. Since it serves as a bridge between the initial safety testing and larger-scale trials that determine overall efficacy. To understand the importance of this trial, it is necessary to delve into its purpose. Other than the procedures, and the vital role it plays in advancing vaccine research.
What is a Phase 2 Vaccine Trial?
A phase 2 trial follows a successful phase 1 trial, which focuses primarily on the safety of a vaccine in a small group of participants. While phase 1 trials involve 20 to 100 people, phase 2 typically includes hundreds of participants. This offers a broader scope for evaluation. The main goal of this stage is to continue assessing the vaccine’s safety, while determining its ability to provoke an immune response.
In a phase 2 trial, researchers examine whether the vaccine produces the desired immune response in the participants and if it can do so consistently. The trial evaluates the appropriate dosage, the number of doses required, and how often they should be administered. It is during this phase that scientists begin to refine their understanding of how the vaccine will work in the broader population.
How Does a Phase 2 Vaccine Trial Work?
In a phase 2 vaccine trial, volunteers are divided into groups, with some receiving the vaccine while others may receive a placebo or a different dose. This allows researchers to compare the outcomes across groups and determine the most effective dose. Transitioning from phase 1 to phase 2 means that the vaccine has already shown promise in terms of safety. However, now it must be tested on a larger and more varied group of participants.
Recruiting a diverse group of participants is essential during the phase 2 trial. Since it provides insight into how the vaccine performs across different demographics, including age, gender, and underlying health conditions. This step is critical because a vaccine must be effective in various segments of the population to ensure its overall success.
Key Objectives of a Phase 2 Vaccine Trial
The objectives of a phase 2 vaccine trial are clear and multifaceted. First, it continues to assess the vaccine’s safety by monitoring any adverse reactions that participants might experience. However, the focus extends beyond just safety; researchers also want to confirm that the vaccine stimulates the immune system in the desired way. This immune response may be measured through blood tests that check for antibodies or other immune markers that indicate a protective effect.
Additionally, the phase 2 trial seeks to refine the optimal dose. A balance must be struck between effectiveness and minimizing side effects. Since a higher dose might provoke a stronger immune response but also increase the likelihood of adverse reactions. Finding the right dose is essential for moving forward with confidence to the next phase.
The Importance of Healthy Volunteers in the Phase 2 Vaccine Trial
One of the fundamental aspects of conducting a successful phase 2 trial is the participation of healthy volunteers. These volunteers are critical because they provide baseline data without the confounding variables introduced by underlying health conditions. Healthy volunteers help researchers gauge the vaccine’s safety in individuals without compromised immune systems. Thus, it is an essential first step before testing on broader populations with varied health statuses.
Healthy volunteers to help approve vaccines are invaluable because they allow researchers to collect data. This in turn will support the vaccine’s approval in subsequent phases. Their participation is essential in evaluating both safety and immune response. Since it directly informs whether the vaccine will proceed to phase 3 trials. Without their willingness to participate, the progress of vaccine development could be significantly delayed.
Monitoring and Data Collection
Throughout the phase 2 vaccine trial, monitoring participants is of paramount importance. Participants are closely observed for any side effects, and data is continuously collected to track the immune response. This data includes information on the vaccine’s ability to produce antibodies. Furthermore, tracking any symptoms or adverse events that might arise after vaccination.
The data collected during this phase is analyzed to determine whether the immune response is consistent across different groups of participants. In addition, researchers look for patterns in any side effects that may occur. Thus, noting whether certain demographics are more susceptible to adverse reactions. Transitioning into the final phases of vaccine trials requires confidence that vaccines can be safely administered to a larger group of people. As a result, this data is key to making that determination.
Challenges in Phase 2 Vaccine Trial
Despite the critical role that such trials play, they are not without challenges. One of the most significant hurdles is recruiting a sufficient number of volunteers who meet the criteria for participation. Trials must ensure that the volunteer pool is diverse enough to reflect the population that the vaccine will ultimately serve.
Phase 2 trials often take place in multiple locations to ensure that data is collected from a wide range of participants. Coordinating these efforts across different regions or countries can introduce logistical challenges including variations in how the trial is conducted or monitored. Despite these obstacles, the data gathered in this phase is critical to ensuring that the vaccine can move forward in the development process.
The Transition to Phase 3
Once a phase 2 vaccine trial is successfully completed, the vaccine moves into the much larger phase 3 trials. These involve thousands or even tens of thousands of participants. Phase 3 trials are designed to definitively prove that the vaccine is both safe and effective across a wide range of people.
Data from these trials helps guide the design of these larger trials by identifying potential issues with dosing, timing, or side effects. If a phase 2 trial reveals any concerning trends, adjustments can be made before moving to the final stage of testing. These adjustments help ensure that phase 3 trials run as smoothly and safely as possible, with minimal risks to participants.
Conclusion
In summary, the phase 2 trial is a crucial step in the vaccine development process. Building on the preliminary safety data collected in phase 1 while refining key elements such as dosing, scheduling, and immune response. The information gathered during this phase is indispensable in determining whether a vaccine is ready to move forward into large-scale trials. Healthy volunteers play a pivotal role in ensuring the success of these trials, as they provide the baseline data needed to evaluate both safety and efficacy.
The insights gained from phase 2 trials help researchers adjust and optimize the vaccine before it reaches the broader population. In any case, ultimately helping to bring safe and effective vaccines to market. As vaccine research continues to evolve, the importance of each phase, particularly the phase 2 vaccine trial, remains clear. Its role in refining and proving the vaccine’s potential cannot be overstated. Thus, marking a critical juncture in the journey towards approval and widespread use.